Abstract
Introduction
Long-term follow up of prospective studies has shown that continuous Bruton's tyrosine kinase inhibitor (BTKi) therapy leads to durable remissions in previously untreated TP53-altered CLL. Additionally, combining BTKi with the BCL2 inhibitor venetoclax (VEN) achieves deep remissions with similar rates of undetectable measurable residual disease in these high-risk patients (pts) compared to those with wildtype TP53. Without randomized data, it is unclear which pts would benefit most from combined targeted therapy over BTKi monotherapy. It is also unknown how size of the TP53-altered clone influences efficacy of BTKi therapy. We performed a retrospective analysis of pts with CLL with baseline deletion 17p [del(17p)] and/or mutated TP53 (TP53-m) treated with BTKi +/- VEN +/- CD20 mAb in the first-line setting.
Methods
Pts with CLL/SLL and pretreatment testing (FISH and TP53 sequencing by NGS assay in >95% of pts with 1% limit of detection) performed at our institution (MDACC) demonstrating del(17p) and/or TP53-m who received first-line BTKi-based therapy were included. Pts started treatment between March 2012 and June 2021. The primary endpoint was progression-free survival (PFS) from therapy start. Pts were categorized into those who received first-line BTKi +/- CD20 mAb versus combined BTKi and fixed-duration VEN +/- CD20 mAb. Outcomes were analyzed by baseline characteristics, including size of the TP53-altered clone (% cells with del(17p) and variant allele frequency [VAF] for TP53-m).
Results
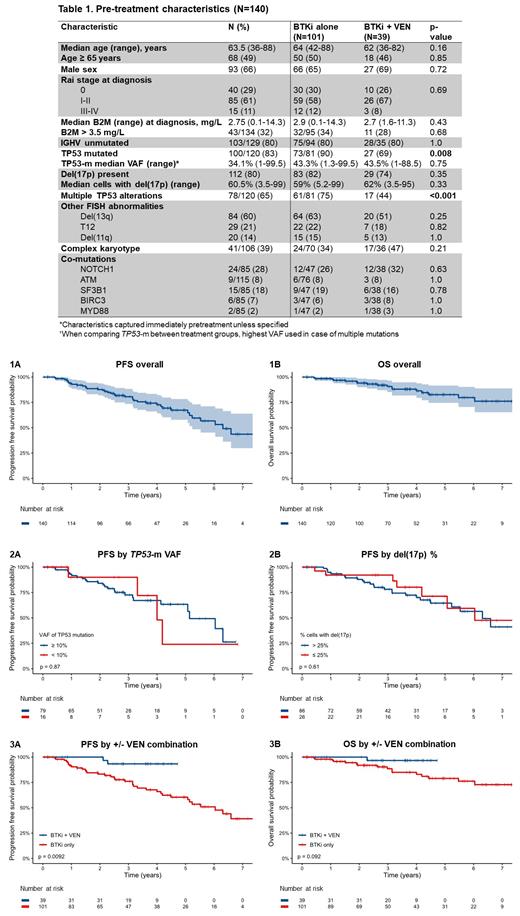
A total of 140 pts with TP53 alteration who received first-line BTKi-based treatment for CLL were included. Pretreatment characteristics are summarized in Table 1. The median follow-up was 3.0 years (range, 0.1 to 9.0). Overall, a total of 38 (27%) pts experienced a progression event, 18 (13%) pts died, and 10 (7%) experienced RT. The 4-year PFS rate was 72.7% and median PFS was 6.3 years (Fig. 1A). The 4-year OS rate was 87.2% (Fig. 1B). A total of 112/140 (80%) pts had pretreatment del(17p). The median % of cells with del(17p) was 60.5% (range, 3.5-99). TP53-m was noted in 100 of the 120 (83%) tested. There were 120 unique TP53 mutations, of which 115 had an available VAF; the median VAF was 34.1% (range, 1-99.5). Among the pts tested for both del(17p) and TP53-m (n=120), 42 (35%) pts had a single alteration [del(17p) or single TP53-m] and 78 (65%) had multiple alterations [both del(17p) and TP53-m or multiple TP53-m]. By univariable analyses, no baseline characteristic was significantly associated with PFS. PFS was not different for TP53-m pts based a VAF threshold of 10% (Fig. 2A) and del(17p) pts based on a threshold of 25% cells affected (Fig. 2B). No baseline characteristic was associated with OS.
A total of 101 (72%) pts received a BTKi +/- CD20 mAb without VEN; 39 (28%) received BTKi and VEN (+/- CD20 mAb) (4 pts included who did not get VEN due to early study withdrawal or data censoring). PFS was significantly longer for BTKi + VEN pts vs. BTKi only pts (p=0.009, Fig. 3A) with a HR of 0.18 (95% CI 0.04-0.77) and there was a trend for longer OS in BTKi + VEN pts (p=0.092, Fig. 3B) with a HR of 0.21 (95% CI 0.03-1.57). BTKi + VEN pts were less likely to harbor TP53-m (69% v 90%, p=0.008) or multi-alteration TP53 (44% v 75%, p<0.001), but no other baseline characteristics were significantly different (Table 1). A total of 28/140 (20%) pts received a CD20 mAb. There was no significant association of CD20 mAb with PFS (p=0.54).
Conclusions
We report favorable 4-year PFS and OS rates of 72.7% and 87.2% in a large retrospective cohort of pts with TP53-altered CLL receiving first-line BTKi-based therapy. The clone size either by % FISH+ or by TP53-m VAF was not associated with PFS, which may reflect the high efficacy of BTKi. PFS appears longer for pts treated with BTK + VEN compared to BTKi only pts, and there was a trend towards improved OS. Though the BTKi + VEN group had lower rates of TP53-m and multiple TP53 alterations, neither characteristic was associated with shorter PFS. Heterogeneity in patient follow-up and response assessment schedule across this pt cohort could be a confounding factor. Whether combination fixed-duration BTKi + VEN is preferable over sequential treatment with these agents remains an unanswered question; this is especially pertinent in pts with high-risk genomics. Therefore, studies dedicated to enrolling such patients are needed to formally clarify optimal treatment for these high-risk patients.
Burger: Beigene: Research Funding, Speakers Bureau; TG Therapeutics: Other: Travel/Accommodations/Expenses, Research Funding, Speakers Bureau; Novartis: Other: Travel/Accommodations/Expenses, Speakers Bureau; AstraZeneca: Consultancy; Gilead: Consultancy, Other: Travel/Accommodations/Expenses, Research Funding, Speakers Bureau; Pharmacyclics LLC: Consultancy, Other: Travel/Accommodations/Expenses, Research Funding, Speakers Bureau; Janssen: Consultancy, Other: Travel/Accommodations/Expenses, Speakers Bureau. Thompson: Genentech: Other: Institution: Advisory/Consultancy, Honoraria, Research Grant/Funding; Amgen: Other: Institution: Honoraria, Research Grant/Funding; Janssen: Consultancy, Honoraria; Pharmacyclics: Other: Institution: Advisory/Consultancy, Honoraria, Research Grant/Funding; Adaptive Biotechnologies: Other: Institution: Advisory/Consultancy, Honoraria, Research Grant/Funding, Expert Testimony; Gilead: Other: Institution: Advisory/Consultancy, Honoraria; AbbVie: Other: Institution: Advisory/Consultancy, Honoraria, Research Grant/Funding. Ferrajoli: Janssen: Other: Advisory Board ; AstraZeneca: Other: Advisory Board, Research Funding; BeiGene: Other: Advisory Board, Research Funding. Sasaki: Daiichi-Sankyo: Membership on an entity's Board of Directors or advisory committees; Pfizer: Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Research Funding. Kantarjian: Immunogen: Research Funding; Aptitude Health: Honoraria; Daiichi-Sankyo: Research Funding; Astra Zeneca: Honoraria; Amgen: Honoraria, Research Funding; Pfizer: Honoraria, Research Funding; NOVA Research: Honoraria; Jazz: Research Funding; KAHR Medical Ltd: Honoraria; BMS: Research Funding; AbbVie: Honoraria, Research Funding; Ipsen Pharmaceuticals: Honoraria; Astellas Health: Honoraria; Ascentage: Research Funding; Novartis: Honoraria, Research Funding; Precision Biosciences: Honoraria; Taiho Pharmaceutical Canada: Honoraria. Wierda: Juno Therapeutics: Research Funding; KITE Pharma: Research Funding; Loxo Oncology, Inc.: Research Funding; Miragen: Research Funding; Cyclacel: Research Funding; Sunesis: Research Funding; Acerta Pharma Inc.: Research Funding; Pharmacyclics LLC, an AbbVie Company: Research Funding; Oncternal Therapeutics, Inc.: Research Funding; Karyopharm: Research Funding; Gilead Sciences: Research Funding; Xencor: Research Funding; Janssen: Research Funding; Genentech: Research Funding; AstraZeneca: Research Funding; GSK/Novartis: Research Funding; Genzyme Corporation: Consultancy; AbbVie: Research Funding. Jain: Beigene: Honoraria; Janssen: Honoraria; Servier: Honoraria, Research Funding; AbbVie: Honoraria, Research Funding; Incyte: Research Funding; Genentech: Honoraria, Research Funding; Precision Biosciences: Honoraria, Research Funding; TG Therapeutics: Honoraria; AstraZeneca: Honoraria, Research Funding; Fate Therapeutics: Research Funding; Pharmacyclics: Research Funding; Cellectis: Honoraria, Research Funding; ADC Therapeutics: Honoraria, Research Funding; Aprea Therapeutics: Research Funding; Pfizer: Research Funding; Adaptive Biotechnologies: Honoraria, Research Funding; Bristol Myers Squibb: Honoraria, Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal